
In the terminology of nonlinear dynamics, the set of subsequences is a sampled reconstructed phase space of the underlying process. Thus, the ith vector u i = ( x i, x i+ 1, …, x i+ m).
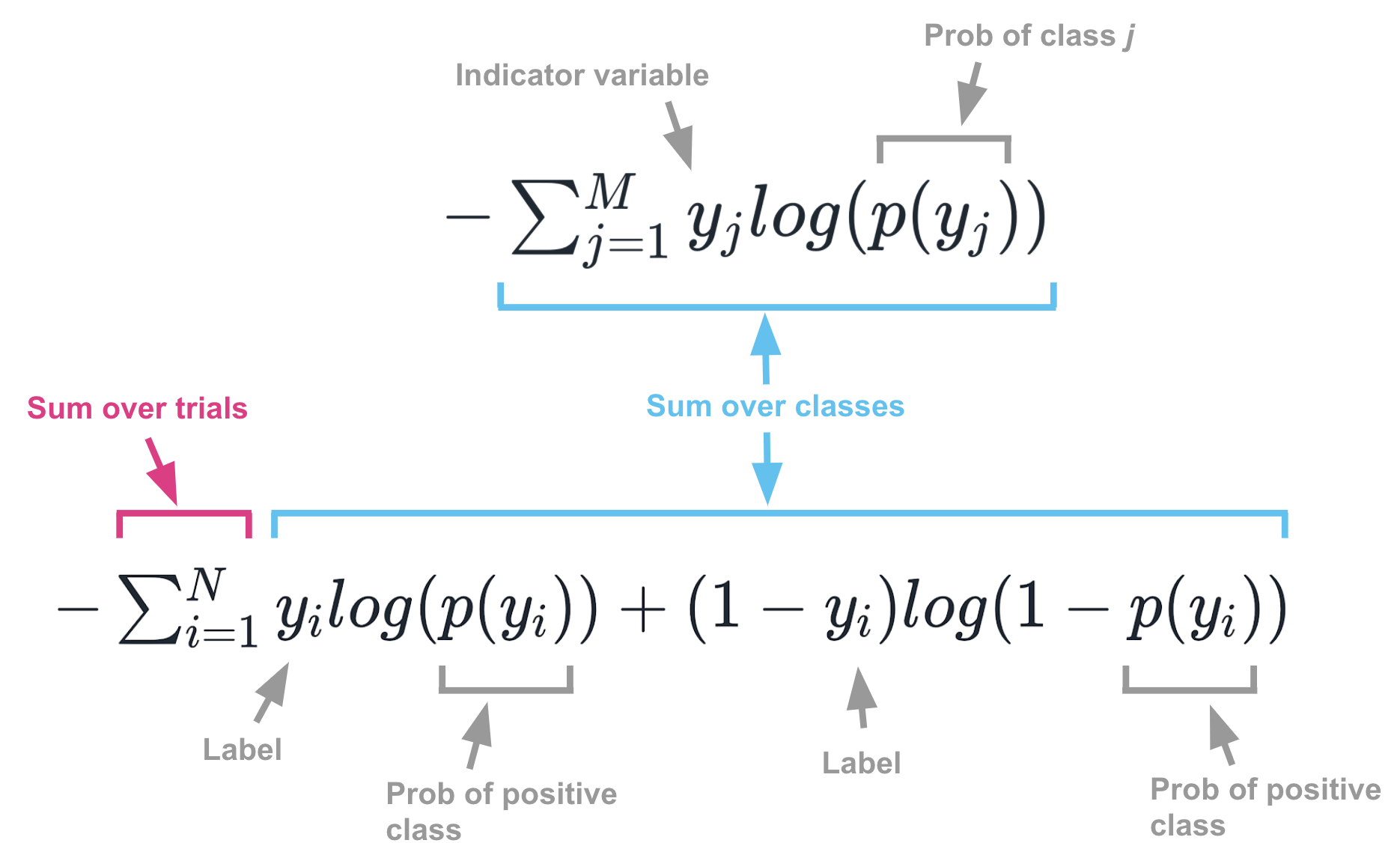
#ENTROPY FORMULA SERIES#
SampEn begins with a time series x, whose ith point is x i and divides it into vectors u i of a specified length m + 1. Richman, in Methods in Enzymology, 2011 7 Relationship Between SampEn and MN-SampEn Some research topics in entropy are the determination of entropy changes in the mixing of very similar species (e.g., isotopes), in very cold systems (i.e., T → 0), and in highly dispersed systems. įor any complete cycle, the change of entropy is zero.Įntropy remains constant for any reversible adiabatic change so that dS = 0.

The entropy of an insulated closed system remains constant in any reversible change, increases in any natural change, and reaches a maximum at equilibrium.

The entropy change of water vaporization at 373.15 K is Δ S v = Δ H v/ T = 108.95 J/(mol K). The entropy change of ice melting at 273.15 K is Δ S m = Δ H m/ T = 21.99 J/(mol K). The entropy of a system is the sum of the entropies of all changes within the system. The change of entropy is expressed as, dS = d e S + d i S where d e S ( d e S = q/ T) is the change due to the interaction of a system with its surroundings, and d i S is the increase due to a natural change, such as a chemical reaction, within the system and is always positive for irreversible changes ( d i S > 0) and zero at equilibrium ( d i S = 0). įor a single phase, dS ≥ q/ T, the inequality is for a natural change, while the equality is for a reversible change.The determination of entropy requires the measured enthalpy and the use of relation T( ∂S/ ∂T) P = ( ∂H/ ∂T) P = C P. Įntropy is a state function and an extensive property.

Some important properties of entropy are: When a fluid system changes from state A to state B by an irreversible process, then the change of its entropy is to be Δ S = S B − S A. Where δq rev is the reversible heat flow.
#ENTROPY FORMULA CODE#
MATLAB code for calculating entropy is available at: These variables are extensively employed in the estimation of the degree of nonlinearity of the systems and correspondence of signals ( Sanei, 2013). Entropy is employed in the calculation of many other useful parameters, such as mutual information, negentropy, nongaussianity and Kulback-Leibler divergence. Noise increases the uncertainty, and noisy signals have higher entropy, even if the original signal is ordered. In this circumstance, the entropy is called conditional entropy.
#ENTROPY FORMULA PDF#
On the other hand, the PDF can be substituted by a conditional PDF in places where the incidence of an event is subject to another event. Thus, usually, the distribution can be a joint PDF when the multichannel signals are jointly processed. Although this measure is utilized for single-channel biomedical signals, it can be simply extended to multichannel or even multidimensional signals. Usually, the distribution can be a joint PDF when the biomedical signal channels are jointly processed.


 0 kommentar(er)
0 kommentar(er)
